Lithium Ores Li2O Spodumene LiAlSi2O6 lepidolite Supply In Nigeria
WE ALSO DEAL IN SUPPLY OF OTHER SOLID MINERALS – Tourmaline, Marble, Tantalite, Feldspar, Kaolin, lithium ore in Nigeria
Lithium Ores Li2O Spodumene LiAlSi2O6 lepidolite
We supply from NIGERIA state from our mine site to any parts of the world. An average 22 Tyre Long Trailer weighs 50 Tons. We can supply as many stones as you need to your country, factory or construction site with Insurance to cover any logistics. Feel free to CALL+ WHATSAPP +234 7057674125, +2348169561788
NOTICE TO ALL BUYERS PAY BEFOE ANY SERVICES, WE DO NOT SUPPLY GOODS ON CREDIT YOU COME TO SITE PAY AND LOAD
NOTICE TO INTERNATIONAL BUYERS: WE ARE 100% RELIABLE DEALERS IN ALL SOLID MINERALS. YOU CAN SEND YOUR REPRESENTATIVE TO NIGERIA TO INSPECT ADN PAY, OR WE CAN RECOMMEND INSPECTION COMPANIES LIKE EYEVIEW, CCIC ETC. VERY RELIABLE AFTER INSPECTION YOU PAY AND YOUR CONTAINERS LOAD GOODS.
WE DO NOT OFFER PAY ON DELIVERY ONLY SERIOUS BUYERS NEEDED
MINERAL IN STOCK SURPLUS QUANTITY AVAILABLE FOR SERIOUS BUYERS
- ILMENITE(TiO2 more than 45%)
- RUTILE(TiO2 more than 60%),
- ZIRCON sands and ore, ZrO2 more than 35%
- Iron ore(Magnetite, TFe more than 58%, FeO more than 20%
- Copper ore, Cu more than 20%
- Nickel ore, Ni more than 5%
- Cobalt ore, Co more than 5%
- Tin ore(cassiterite), Sn more than 50%
- Rare earth ore, monazite ore, ReO more than 30%
- Tungsten ore, W more than 5%
- Molybdenum ore , Mo more than 5%
- Antimony ore, Sb more than 30%
- Lead ore(galena etc.)Pb more than 50%
- Zinc ore (Zn more than 45%)
- Coltan ore (Ta2O5 more than 30%),Niobium ore
- lithium ore(spodumene ),Li2O 1% to-more than 5%
- Manganese ore(but Mn must be more than 50%)
etc. - Lepidolite/ Kunzite/ Spodemene= lithium
- Then White Dolomite
- Finally Amblygonite( lithium)
- quartzites
- Tantalite
- Columbite
- Cassiterite
- Bismuth
- Gemstones
- Gold
- Marble
- Red clay
- Sand
- Gravel
- Granite
- Syenite
- kaolin
- Talc.

Lithium Ore Supply In Nigeria
Are you Looking to buy Lithium? our team of Miners, we explore, blast, mine, supply, and export Lithium Ore. Our ideology basically is ‘Alliance with Local and International Bodies’ which was inspired to provide value-added services in the minerals Markets. Whether you want to purchase Lithium Ore locally or have it shipped to any port around the world, our world-class team is built to help you close fast, safe, and profitable transactions on time, every time!
Product Specifications:
| Li | 5% Min up to 10% |
| AI2O3 | 30% Max |
| SIO2 | 62% Max |
| Cl | 0.05 Max |
| Fe2O3 | 2% Max |
| Mineral Type: | Lithium Ore / Lepidolite / Amblygonite / Spodumene / Petalite |
| Physical Size | Depend on Buyers request / 0-15 mm or 10-150 mm |
| Packaging | 50KG PP bags, 1 Ton Big bags or Bulk in Container |
| Stuffing | Max 28MT per 20FT Container |
| Quantity | 500 MT per month |
| Country of Origin | Nigeria |
| Loading Port | Lagos Ports or Port Harcourt Ports |
| Loading Time | Within 30 days upon receipt of LC confirmation/BG/SBLC/APG |
Inspection at the ports by SGS / CCIC / Eyeview / Bureau Veritas /

Lithium Ores Spodumene and lepidolite Supply In Nigeria
Concerns about increasing atmospheric CO2 concentrations and its adverse effects on global climate have led to an increased use of renewable energy such as solar, wind and wave, and consequently an increased requirement to store electrical energy. Due to the high energy density of lithium, recent studies focus on its application in future technologies in order to meet the world’s increasing energy demands (Fergus, 2010; Hu et al., 2013; Kucinskis et al., 2013; Liu et al., 2014; Manthiram et al., 2014; Scrosati and Garche, 2010; Stephenson et al., 2014; Tarascon and Armand, 2001; Wild et al., 2015). Rechargeable Li-ion batteries are the preferred energy storage technology in both consumer electronics as well as electric vehicles (EVs), resulting in continuously increasing demand for lithium. The total annual lithium demand has been increasing at a rate of 6 % to 9 % pa between 2015 and 2020 (Martin et al., 2017b).
The significant environmental and economic cost of refining lithium from pegmatites and the increase in lithium price over the last two years open a window of opportunity to develop innovative metallurgical processes. Forecasts suggest decreasing price for lithium chemicals falling by around 45 % by 2021 from their peak values in 2017 and early 2018 (Priscila, 2018). These processes need to enable more efficient and more environmentally benign lithium extraction from spodumene and other pegmatites while minimising the amount of chemical feedstocks, by-product formation and energy requirements and thus the environmental footprint and cost of production of lithium chemicals. Besides salar brines, silicate minerals, such as spodumene LiAlSi2O6, constitute the most important source of lithium (Chagnes and Światowska, 2015; Garrett, 2004; Habashi, 1969). The large resources of spodumene and lower grade lithium ores such as petalite LiAlSi4O10, lepidolite K(Li,Al)3(Si,Al)4O10(F,OH)2, or zinnwaldite KLiFe2+Al(AlSi3O10)(F,OH)2 promise to satisfy the increased demand for lithium and respond rapidly to fluctuations in market demand. Extraction of Li2CO3 from brines relies on slow evaporation that requires long lead times, in the order of two years, in comparison to five days needed for refining lithium chemicals from pegmatites (Chagnes and Światowska, 2015; Jiankang et al., 2015; Yaksic and Tilton, 2009). However, the production of battery-grade lithium carbonate from silicate sources such as spodumene is generally more complex and energy-intensive than the production from brines, often resulting in doubling of the production costs (Chagnes and Światowska, 2015). Spodumene represents the most abundant and economically most important mineral among lithium pegmatites and clays, with spodumene containing up to 8 wt. % Li2O (Edgar, 1968; Kesler et al., 2012; Vikström et al., 2013).
This has motivated the utilisation of this valuable resource to meet the accelerating and fluctuating demand for lithium. The naturally occurring spodumene adopts the α-structure (α-LiAlSi2O6), i.e., the stable lowtemperature form (Clarke and Spink, 1969). This phase exhibits a dense and fully ordered monoclinic (C2/c) configuration, resulting in high resistance towards attacks by chemical agents (Rosales et al., 2014). The current modus operandi recovers lithium from spodumene via acid digestion (Chagnes and Światowska, 2015), which requires initial thermal treatment, i.e., the transformation of α-spodumene into more reactive phases that are amenable to digestion by strong sulfuric acid. At high temperatures, spodumene transforms into tetragonal β- and the hexagonally structured γ-phase (Li, 1968; Li and Peacor, 1968). These polymorphs relate to one another by reconstructive transformations (Li, 1968) that are associated with a wide temperature window, slow kinetics and considerable energy consumption (Putnis, 1992).
This transformation process of α- to β-spodumene underpins the subsequent ion exchange of Li+ with H + to produce lithium chemicals (Clarke and Spink, 1969; Rosales et al., 2014; Skinner and Evans, 1960; Tian et al., 2011; White and McVay, 1958). The heat treatment of spodumene, that, is usually performed in a rotary kiln, consumes large amounts of energy (Garrett, 2004; Metsärinta, 2015; Peltosaari et al., 2016). The aggressive thermal and acidic treatments of spodumene, in addition to purification and recycling of by-products, constitute the key factors that define the environmental footprint of extraction of lithium from on an industrial scale. Recent reviews on lithium processing provided a general explanation about producing lithium chemicals from lithium minerals, brines, seawater and spent lithium-ion batteries (Choubey et al., 2017; Choubey et al., 2016; Meshram et al., 2014; Swain, 2017).
A detailed review that particularly focuses on spodumene processing has not been reported in the literature. For this reason, this review comprises a comprehensive summary of the processing technologies of spodumene concentrate, including the associated thermal analysis of its phase transition. The present review is in three parts. The first part focuses on the importance, usage and demand for lithium, addressing how processing of spodumene responds to these factors. The second part characterises the structural transformation of spodumene. Particularly, it focuses on mineralogical, thermodynamic and kinetic aspects that arise during calcination of spodumene, as presently practiced by industry. The last part summarises the known technologies that can produce valuable lithium compounds from spodumene, as suggested in literature or implemented in industrial operations. This review describes in details the spodumene processing routes, concentrating on methods of sulfate roasting and digestion by sulfuric acid and proposing an approach that we later develop in this thesis. The beneficiation of spodumene ore and the purification of the pregnant leach solution (PLS) fall outside the scope of this review. From time to time, we make references to the processing of lepidolite, for the reader to gain an improved understanding of treating spodumene. However, an exhaustive description of technologies for recovering lithium chemicals from lepidolite, per se, is also outside the scope of this review.
Concerns about the carbon dioxide footprint of the transport industry and also about the reliance on oil imports have attracted more interest to the electric vehicles (Vikström et al., 2013). In 1992, the nickel batteries, nickel-cadmium and metal hydride (NiMH), started to be replaced by lithium-ion batteries (LIBs) as reflected in Figure 2.2. While the NiMH batteries demonstrated a satisfactory performance in EVs with reasonable thermal stability and energy density (Goonan, 2012), the replacement of NiMH by LIBs occurred because of the capacity of LIBs to reach energy density in excess of 140 Wh kg-1 , much higher in comparison to 55 Wh kg-1 of nickel batteries (Shukla and Kumar, 2013). Their safety properties signify the main advantage of the NiMH battery systems.
Data are presented as an annual percentage of the total sales of different rechargeable batteries. After Goonan, 2012. Table 2.1 presents a comparison between the NiMH and lithium-ion batteries. The trend in the replacement of the NiMH batteries by LIBs in the electronic devices prompted the adoption of LIBs in electric vehicles manufactured after 2008 (Goonan, 2012). Importantly, beside the higher energy density of the LIBs, they have the capacity for recharging even after being fully depleted (Gaines and Cuenca, 2000; Shukla and Kumar, 2013; Vikström et al., 2013). The EVs currently average around 3 % of the annual total vehicle production, but they are expected to reach about 52 % in 2030 and 90 % in 2050 (Goonan, 2012). Thus, sales of EVs drive the global demand for lithium.
IEA’s estimate relies on its blue map scenario in which IEA suggested that, the development of new energy technologies would reduce the annual global CO2 emissions in 2050 to half of the level of 2005. Beside the lithium demand for EVs, other uses for lithium are likely to increase as well. Thus, the total consumption of lithium may exceed the IEA’s estimate (Vikström et al., 2013). Igneous lithium rocks and brines constitute the most abundant sources of lithium (Chagnes and Światowska, 2015). Processing of brines involves evaporation, ion exchange, precipitation, solvent extraction and adsorption (Garrett, 2004). Chile and the USA possess the largest resources of lithium in brines (Chapter 8, Appendix 1 Table S2.1).
Lithium in Nigeria
Lithium is a highly reactive metal that is used for making energy-dense rechargeable batteries for electronics, such as laptops, cell phones, electric vehicles, and grid storage.
“Nigeria lithium is hot cake now, the mineral was discovered in several parts of the country in the course of the National Integrated Mineral Exploration Project during exploration and we did investigation and came up with analysis and discovered that Nigeria lithium is of high grade.
He added, “High grade in the sense that the standard worldwide for even exploration and mining starts from 0.4 per cent lithium oxide but when we started exploration and mining, we saw one per cent up to 13 per cent lithium oxide content.
“Another advantage of Nigerian lithium is that it is hard rock lithium and that is what investors are looking out for worldwide. We even had discussions with some private companies with mining licences in Nigeria and they conducted tests on the lithium and we also collaborated with Canada on that too.”
He said that a track of mineralisation almost two to 10km was discovered close to Abuja, adding that resource evaluation was conducted on the area and anomolous gold, lead and silver were discovered with Tungsten, an addictive for steel production.
six zones in Nigeria; we have been able to produce lithium.
Is Lithium Found in Nigeria?
The first and most important point is that the discovery does not equate to a commercial find. In fact, it should be taken only as a first step in the long journey to be established as a commercially viable deposit that can be mined and extracted to a form that can be sold to the consumer. In reality only one or two of hundreds of such ‘discoveries’ (finds) may end up being a mine after going through the many stages of exploration and development. Only then can a value be attached to such a ‘discovery’. It can take 5 – 10 years to fully explore a small to medium sized deposit and take it to production. This is provided there are no unforeseen technical, financial and other challenges. Only in few very exceptional and highly viable situations we can expect a lesser time frame. Quite a number of factors can make or mar the development journey. These include proving economic grades and volumes (or tonnages) of the mineral ores, favourable enabling fiscal and other regulatory environments, cost of extraction technology, market forces and other logistical and sociopolitical issues. An economic amount of lithium metal is known to be associated with two minerals: spodumene and lepidolite. Lithium is an element and in nature tends to concentrate sufficiently in the two minerals, spodumene and lepidolite. Otherwise it will occur dispersed in minerals but not sufficient enough to be of economic consideration. They are usually found in specialised rocks called rare metal-bearing pegmatites and greisens. The search for economic deposits of lithium has to be targeted in these rocks. In Nigeria, lithium minerals (spodumene and lepidolite) are known to be associated with cassiterite, columbite-tantalite (coltan) and others in the extensive belt of rare metal-bearing rock types called pegmatite. These rock pegmatites stretch from the Wamba area, Nasarawa State, north central; through Egbe-Isanlu, Kogi State; north central, Ondo-Ekiti States, south west; to the Ife-Ilesa, Osun State; south west. Another belt in the western half of Nigeria, stretching from Zamfara and Kaduna States, north west; through Niger and Kwara States, north central; and Oyo State, south west; is known to host the rare metal-bearing pegmatites. Some have also been found in Obudu, Cross River State, southern Nigeria. Within these belts, dozens of occurrences of the rare metal-bearing pegmatites are recorded and some are known to have lithium-bearing minerals. However, there are not yet any commercially viable deposits established or developed. This is despite the recent wave of interest shown by many explorers in response to the global demand for lithium.
The electric vehicle industry has grown exponentially in recent years. Growth forecasts from BloombergNEF see the share of electric vehicle sales rising from 2 percent in 2018 to 35 percent in 2030.
But the growth will depend on strong supply chains, which rely on a secure and sustainable supply of raw materials, specifically minerals, and metals most of which are in large supply in Nigeria but are grossly underutilised.
Lithium ore, Nickel ore, and Cobalt, the three raw materials required for batteries used to power electric vehicles are found in large commercial quantities in different Nigerian states.
Lithium ore is found in large quantities in Nigerian states such as Kogi, Nasarawa, Ekiti, Kwara, Cross River, Oyo, Plateau, and a few other states. Recently, Gbenga Okunlola, a Professor of Geology at the University of Ibadan, in research submitted to the World Bank, announced the discovery of over 3000 lithium pegmatite bodies across the country. Pegmatite lithium deposits, also known as hard-rock lithium deposits, can contain a number of elements, including lithium, tin, tantalum, and niobium.
There are also preliminary results that have shown that the grades of Nigerian Lithium bearing ores are comparable with grades obtained in lithium-producing mines across the world.
China still continues to dominate the lithium-ion battery supply chain ranking due to continued investment and strong local and domestic demand for its lithium-ion batteries. The Asian nation hosts 80 percent of all battery cell manufacturing capacity today, with capacity expected to more than double, enough for more than 20 million electric vehicles, in the next five years, the BloombergNEF report said.
In Nigeria, the Nigerian Geological Survey Agency (NGSA) has done some level of exploration in lithium but experts say research needs to be conducted at par with the advanced countries.
GLOBAL
The enrichment of lithium from brine deposits requires solar evaporation (An et al., 2012). The evaporation process proceeds in shallow pond systems of a large surface area with exposure to sunlight. While this process is relatively cheap, it is also slow. The lithium production cycle to obtain Li2CO3 may take 1 – 2 years (Vikström et al., 2013) and is too sluggish to match changes in market demand for this commodity. These limitations might be overcome in future by the development of advanced membrane-based purification technologies, but their cost should be optimised (Somrani et al., 2013). Contrary to the evaporation issues in brines, extraction of lithium from rocks (mainly spodumene LiAlSi2O6) requires only around five days, allowing lithium chemicals to be continuously produced through the whole year (Grosjean et al., 2012). Spodumene can be treated equally effectively to obtain LiOH·H2O and Li2CO3 (Chagnes and Światowska, 2015). However, converting Li2CO3 to LiOH involves a reaction with hydrated lime (aka slaked lime or Ca(OH)2) in a relatively complex process (Hader et al., 1951) that is associated with long reaction time and low yield (Yuan et al., 2017).
Among many lithium ores, spodumene occurs in large but non-concentrated deposits (typically 12- 30 % spodumene) in association with quartz (22 – 27 %), feldspar (30 – 50 %) and mica (3 – 5 %), with accessory minerals of apatite, tourmaline and beryl, in addition to traces of rutile (TiO2), cassiterite (SnO2), monazite (Ce,La,Nd,Th)PO4, columbite (Fe2Nb2O6), pyrite (FeS2), and pyrrhotite (FeS) (Colton, 1957). The aluminosilicate pegmatites such as spodumene, lepidolite and petalite are economically viable for lithium processing because of large deposits of these minerals (Meshram et al., 2014). Spodumene represents the most commonly available lithium-bearing pegmatite with high lithium content (Chapter 8, Appendix 1 Table S2.2 and S2.3).
While spodumene mineral may contain up to 8 % Li2O, in practice spodumene mines sell 5.5 – 6 % concentrates, obtained by dense media separation and/or mineral flotation (Chagnes and Światowska, 2015; Garrett, 2004) depending on whether spodumene is primary or secondary (i.e., formed from petalite). Talison Lithium Ltd., the largest producer of spodumene, operates the Greenbushes mine in Western Australia, a joint venture between Tianqi (51 %) and Albemarle Corporation (49 %). Currently, the mine supplies about 30 % of the global demand for lithium (Smith, 2018). The treatment of spodumene in a typical lithium refinery that processes 6 % Li2O concentrate includes calcination, acid digestion, leaching and purification (Garrett, 2004). In the next sections of this Chapter, we will focus on the calcination, digestion and leaching processes, while the purification falls outside the scope of this review. We will also review historical technologies for roasting of spodumene with additives.
Abstract
This work provides a detailed description of the qualitative and quantitative mineralogical, dynamic, as well as kinetic aspects for the structural transformation of α-spodumene (αLiAlSi2O6), and advances the industrial processing of spodumene by introducing two novel alternative technologies that are relatively straightforward and potentially cost-effective. Spodumene, the most abundant lithium-containing mineral, usually undergoes calcination at an extreme temperature of about 1100 ºC and strong-acid digestion during industrial processing. The calcination process stimulates the structural transformation of spodumene from its naturally occurring pyroxene-framework α-phase into the relatively more reactive βspodumene of the keatite (SiO2) structure. On the other hand, the acid digestion approach facilitates the production of water-soluble lithium compounds (mainly lithium sulfate Li2SO4). This study resolves the technical obstacles associated with cheaper (and safer) processing of spodumene concentrates.
2.2. Lithium Importance, Usage and Demand
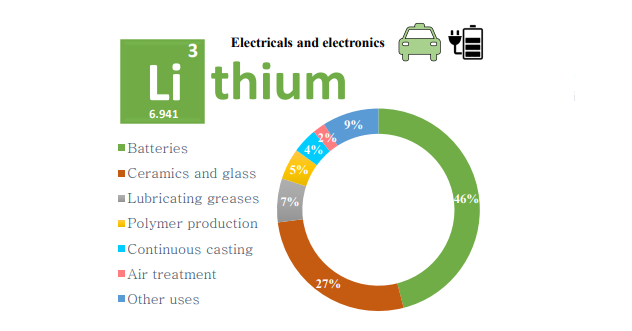
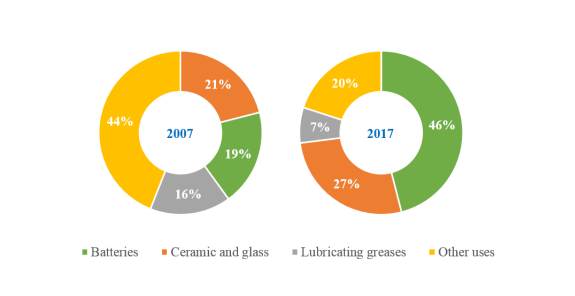
Lithium is the lightest known element that has a high electrochemical reactivity. These properties facilitate significant applications of lithium in energy storage and electric vehicles. The high standard electrochemical potential of lithium affords this element a valuable application in lithium-ion batteries (LIBs). The consumption of lithium in the manufacturing of batteries increased from 19 to 46 % of the global lithium production between 2007 and 2017 and is expected to rise to around 66 % by 2025 (i.e., out of the global lithium production) (Ober, 2008, 2018; Swain, 2017). Lithium compounds have also been widely applied in production of ceramics and glass, lubricating greases, and some other fields such as continuous casting, air treatment, polymers, pharmaceuticals, metallurgy, rubber and thermoplastics (Deberlitz, 2006; Ebensperger et al., 2005; Reichel et al., 2017; Swain, 2017). Figure 2.1 illustrates the distribution of lithium consumption during the decade of 2007 to 2017 in different markets, reflecting a significant increase in the demand of lithium for manufacturing of batteries.
The project incorporated intensive experiments to analyse the thermally-activated changes during the calcination of spodumene. The combination of hot-stage and high-temperature synchrotron X-ray diffractometry (XRD) enabled in-situ mineralogical analysis of the transformation processes, identifying (and quantifying) the resulting phases at various temperatures. Each of the diffractometry techniques complements the heating rate and temperature limitations of each other. Likewise, accurate calorimetric and thermogravimetric analyses yielded the corresponding thermodynamic and kinetic functions, allowing the precise determination of the minimum energy required for the heat treatment process. Distinctly, the project also involved detailed investigation on roasting of spodumene with the most effective vii additives, CaO and Na2SO4, for better extraction of lithium. The addition of these chemicals resulted in the formation of water-soluble lithium compounds via the roasting process at a relatively low temperature (800 – 900 ºC). Set of experiments determined the best condition for minimising these additives and maximising the productivity of lithium. Atomic absorption spectrometry (AAS) quantitated the recovered lithium from the roasted spodumene concentrate. Techniques, such as X-ray fluorescence (XRF) and AAS, attested the chemical analyses of the raw spodumene concentrate.
The Match! Software allowed phase identification, while HSC 7.1 software facilitated the estimation of energies. The results of this thesis have demonstrated that the transition reaction of spodumene occurs via different pathways, depending on the amorphicity and the thermal history of the mineral. The results have also identified the intermediate species and clarified their appearance as a function of temperature and heating rate, and particle size, relative to the final phase of βspodumene.
For instance, the formation of the recently reported γ-spodumene is initiated by crystallisation of minuscule amorphous materials in the concentrated sample at slow heating conditions, while fast initial heating to 800 ºC prompts the emergence of a newly-identified phase of β-quartzss, at low temperatures of less than 900 ºC. Requiring an operating temperature of above 1000 ºC, the calcination of spodumene concentrate has been elucidated to adopt slow kinetics, with a high activation energy of more than 800 kJ mol-1 and significant dependency on the degree of conversion. The combined outcomes of this study are instrumental in optimising the energy cost of lithium extraction from spodumene mineral in practical operations. In particular, this thesis reveals that, the roasting of spodumene concentrate with a small amount of CaO reduces the transformation temperature by 150 – 200 ºC as determined by in-situ XRD, which translates viii into important energy saving during the calcination of spodumene in the first step of the commercial acid digestion process. Roasting of spodumene with CaO and Na2SO4 at 882 ºC for 2 h results in producing a water-leachable lithium compound of LiNaSO4 with 94 % lithium recovery. Thus, the roasting of spodumene concentrate with these two additives eliminates the aggressive acidic treatment and decreases the operating temperature of the kiln. 1.1. Introductory Overture Lithium (Li) symbolises the lightest metal (and solid element) with an exceptional electrochemical reactivity.
As illustrated in Figure 1.1, these properties facilitate significant applications of this element in energy storage and electrical mobility. The mass production of lithium-ion batteries (LIBs) accounted for half of the entire consumption of lithium in 2017 (Ober, 2018). Moreover, rechargeable Li-ion batteries remain the preferable energy storage technology in both consumer electronics and electric vehicles (EVs), resulting in continuously increasing demand for lithium, with the projected compound annual growth rates (CAGR) of 22 – 30 % only in the EVs sector, between 2016 and 2030 (Azevedo et al., 2018), as lithium continues to fuel the technological advancements in the present society. Salar brines and spodumene ores represent the most important sources of terrestrial lithium (Habashi, 1969; Ingham, 2012). Although processing of brines is relatively inexpensive, the lithium production cycle by evaporation requires about two years to complete (Vikström et al., 2013). This undoubtedly attenuates the availability of lithium chemicals and disrupts their accelerating demand (Choubey et al., 2016).
On the other hand, extraction of lithium from spodumene demands approximately five days and affords a year-round production (Grosjean et al., 2012). The abundant deposits of spodumene promise to satisfy the increasing demand for lithium on a rapid time scale, adjusting its availability to market demand. However, the challenge of refining spodumene is that of construction and operation of largescale mines and refineries that entail complex and energy-intensive activities, as compared to those of processing of brines (Chagnes and Światowska, 2015). These activities involve mining, comminution, beneficiation, calcination, acid digestion, leaching and purification; the last step of varying intensity, as mandated by the required purity of the end-use application. In practice, calcination, acid digestion and purification represent the most critical stages for the overall consumption of energy and formation of by-products that are difficult to recycle, especially acidic aluminosilicates and sodium sulfate.
The majority of the published work on spodumene focused on the extraction of lithium from spodumene by acid digestion, alkaline pressure leaching and roasting with additives (Chagnes and Światowska, 2015). In practice, only the digestion of spodumene with sulfuric acid is currently practiced in the production of lithium chemicals on an industrial scale. The concentrated sulfuric acid digests β-spodumene at 250 – 300 ºC for 1 h (Ellestad and Milne, 1950; Tian et al., 2011). Prior to digestion with sulfuric acid, this technology involves a calcination step to convert the acid-resistant α-spodumene to obtain the more reactive β-phase, usually performed at 1050 – 1100 ºC for 1 – 2 h, since α-spodumene, as a pyroxene-group mineral, exhibits extreme lack of reactivity. The processing steps that follow the digestion of the calcined material command feedstocks of CaCO3, Ca(OH)2, Na2CO3 or NaOH to neutralise the acidity of the leachate and precipitate Li2CO3 (Kuang et al., 2018). The purity of these feedstock chemicals matters and often one prefers more expansive NaOH rather than cheaper but less pure CaCO3. Hydrometallurgical processes based on the alkaline pressure leaching of β-spodumene provide an alternative to the present state-of-art technology based on digestion of β-spodumene with concentrated sulfuric acid. In these processes, solutions of Na2CO3, NaCl, NaSO4 leach βspodumene under 20 – 40 bar and 150 – 250 ºC (Chen et al., 2011; Gabra et al., 1975; Kuang et al., 2018).
The pressure-leaching processes operate under alkaline conditions to avoid polymerisation of silica that prompts the formation of impermeable skins on surfaces of particles of β-spodumene. While some recent literature argues for reconsidering the use of the alkaline leaching process on the industrial scale (Choubey et al., 2016; Dlugogorski, 2018), the problem of high energy consumption during the calcination step remains unsolved. Roasting of α-spodumene with salts or oxides of alkali and alkali earth metals may constitute a viable approach to improve the sulfuric acid digestion process that constitutes the present industrial standard. In this approach, compounds of calcium, sodium and potassium serve as additives in the roasting of α-spodumene at elevated temperatures of up to 1100 ºC (Medina and El-Naggar, 1984; Sternberg et al., 1946; Victor, 1953).
High processing temperature, reduction of the spodumene fraction in the feed to a kiln (and hence the process throughput) and melting of excess additives constitute the chief factors that generally hinder implementation of this approach in the industry. However, careful selection of additives, especially combining CaSO4 and NaSO4 with CaO and Ca(OH)2, allows operations at lower temperatures (850 – 1000 ºC). The viability of this process has been demonstrated for refining lithium micas to lithium chemicals. The process exhibits an additional benefit of avoiding digestion or leaching with acids as contacting of the roasted material with water alone displays high recovery of lithium under atmospheric conditions (Jandová et al., 2009; Yan et al., 2012). As the calcination step represents one of the most crucial factors in the processing of spodumene, a great deal of research has been devoted to its understanding and optimisation. Pertinent research has mainly focused on the transformation of α-spodumene to its hightemperature polymorphs. But, the published literature has noted inconsistent results on the transition pathways, that is, the reaction corridors from α- to β-spodumene (Botto et al., 1975; Gasalla and Pereira, 1990; Tian et al., 2011; White and McVay, 1958) and the appearance of γ-spodumene as an intermediate phase. In particular, it remains uncertain whether γspodumene arises along sequential or parallel reaction pathways that lead from α- to βspodumene (Moore et al., 2018; Peltosaari et al., 2015; Salakjani et al., 2016).
A large degree of uncertainty still prevails in the governing kinetic parameters, especially at temperatures exceeding 930 ºC (Botto and Arazi, 1975; Moore et al., 2018). This thesis combines various scientific investigations pertaining to the practical aspects of the calcination and roasting of high-grade concentrates of spodumene from the Talison Lithium mine in Greenbushes in Western Australia and suggests two alternative methods to extract lithium from α-spodumene under relatively less aggressive conditions. (In the thesis, we distinguish between calcination, to denote heating of α-spodumene by itself, and roasting, to signify a thermal treatment of mixtures of α-spodumene with additives. Occasionally, the term decrepitation is introduced to convey the same meaning as calcination). The present work also aims at providing vital mineralogical, thermodynamic and kinetic data. The underlying aim is to build an energy-effective strategy for calcination of α-spodumene through approaches that can be adopted by industry. Along the same line of enquiry, this work targets improvements to the present calcination step by introducing additives that act as fluxes to decrease the calcination temperature or allow avoiding the acid digestion step, by proceeding directly to water leach.
Research Motivation The environmental concerns arising from the carbon footprints of combustion of fossil fuels (e.g., used in automobiles) have prompted the fast development of electric vehicles (EVs) (Vikström et al., 2013). Lithium-ion batteries (LIBs) perform well for applications in EVs, with specific energy now reaching 200 – 260 Wh kg-1 (Choubey et al., 2017; Jain, 2018). As a matter of fact, the recently announced Chinese support for manufacturing of electric vehicles allows 20 % more subsidy for vehicles equipped with battery packs that display energy density of over 140 Wh kg-1 , but 40 % less subsidy for vehicles with batteries characterised by energy density of 105 – 120 Wh kg-1 , in comparison to batteries carrying 120 – 140 Wh kg-1 . Vehicles equipped with battery packs that display energy density lower than 105 Wh kg-1 receive no subsidy (Hu and Yuan, 2018). The EVs currently average 3 % of the annual total vehicle production, but are expected to reach about 52 % in 2030 and 90 – 95 % in 2050 (Goonan, 2012; Sarkar et al., 2018). Moreover, storage of renewable energy requires large battery capacity, with LIBs competing effectively against other battery technologies. The largest lithium battery in Australia of Hornsdale Power Reserve of 100 MW/129 MWh entered into operation at Jamestown, South Australia (Hendriks, 2018), providing a network of security services to South Australian electricity consumers.
This paired energy storage system of Tesla and Neoen Wind sets the path for global solution of the future grid-scale battery storage (Deloitte, 2018). Forecasts of a significant increase in the global demand for lithium chemicals have prompted rapid development of spodumene mining and refining in Western Australia, swiftly adding to the spodumene production coming from the Talison Lithium mine in Greenbushes located in the South West region of WA. In 2017, the Greenbushes mine delivered 646 kt of ca 6 % spodumene concentrate that corresponded to 30 % of the global demand for lithium, with plans to expand the production to 2.3 Mt of spodumene concentrate (Smith, 2018). Over the last two years, three new spodumene mines in Pilbara region, in the north of Western Australia, have commenced shipping the run-of-mine (ROM) ore or spodumene concentrate (Pilbara Resources and Altura Mining, both attached to the Pilgangoora deposit, and Mineral Resources exploiting the Wodgina deposit), and two in the Goldfields (Tawana Resources mining the Bald Hill deposit and the joint venture of Mineral Resources, Neometals and Jiangxi Ganfeng Lithium exploiting the Mt Marion spodumene).
After two and a half years of shutdown, in 2015, the Galaxy Resources re-opened its Mt Cattlin spodumene mine that operates near Ravensthorpe, also in the Goldfields region of WA. In total, seven mines are presently producing ROM ore or spodumene concentrate in WA (Hastie, 2018). The final entry into the lithium mining and refining is Covalent Lithium, the 50/50 joint venture of Kidman Resources and Sociedad Química y Minera de Chile (SQM), that is proceeding to exploit its Mt Holland deposits in the Goldfields and build a lithium refinery in Kwinana, south of Perth. Kwinana will also host a lithium refinery presently in construction by Tianqi Lithium
2.4. Metallurgical Processing of Spodumene
In many cases in the extraction of lithium from β-spodumene, less strict conditions result in a higher conversion. Three technologies dominate processing of spodumene: (1) acid route for digesting of β-spodumene with concentrated H2SO4 (> 90 wt.%) at 200 – 300 ºC to produce the water-soluble Li2SO4; (2) roasting route for heating of spodumene (mainly α-spodumene) with alkali compounds (alkaline oxides, hydroxides, carbonates, chlorides and sulfates) at high temperatures to produce soluble lithium compounds; (3) alkaline leaching route for reacting β-spodumene with solutions of salts and hydroxides of alkali and alkali-earth metals at high pressure and moderate temperatures (40 bar, 250 ºC) to produce soluble lithium compounds.
Comminution indicates the reduction of spodumene particle sizes by crushing and grinding; Beneficiation denotes the process of removing the associated gangue minerals (impurities) from spodumene ore to produce a higher grade concentrate; Calcination/Decrepitation corresponds to heating of α-spodumene concentrate at 1050 – 1100 ºC to engender the structural transformation of α- to β-spodumene; Acid digestion signifies the strong acid treatment of spodumene with mainly H2SO4 under thermal conditions of 250 – 300 ºC to produce the water-soluble Li2SO4; Roasting stands for the heating process of spodumene with alkali and alkali-earth metal compounds (salts, oxides and hydroxides) at high temperatures to produce soluble lithium compounds; Leaching is the dissolution of solvable lithium species by hot water or extraction of lithium from β-spodumene by solutions of salts and hydroxides during a high-pressure leach operation; Alkaline leaching involves highpressure reaction of β-spodumene (40 bar, 250 ºC) with solutions of (mainly) sodium salts such as Na2CO3, Na2SO4 and NaCl under alkaline conditions.
Two technologies have been used for spodumene processing on industrial scale, namely: (1) lime roasting process, the method that was implemented by the Foote Mineral Company in the USA (now Chemetall-Germany) 1950 – 1984 for roasting of spodumene with CaO and producing LiOH. This technology could not compete, especially in the late 1990s, when the South American producers of Li2CO3 from brines decreased their prices. (2) The sulfuric acid digestion process is the only technology presently available to refine β-spodumene to lithium chemicals. Metalloy Corporation, subsequently a division of Lithium Corporation of America then FMC Lithium and now Livent Corporation, introduced the sulfuric acid digestion process in the 1940s (Ellestad and Milne, 1950; Hader et al., 1951), presently implemented in all lithium refineries across the globe.
For example, by Jiangsu (China) deploys it to produce Li2CO3 or Nemaska Lithium Inc to manufacture high purity LiOH of 99.7 %. Recently, Tianqi Lithium Australia has located its lithium refinery in Kwinana, Western Australia, to produce battery-grade LiOH with a design capacity of 48,000 tonnes per year (almost 60 times larger than the operation of Lithium Corporation of America at its original plant in St. Louis Park, Minnesota, USA, ca 1951!), to come on stream in the second half of 2019 (Vaughan et al., 2018). Five other companies are either conducting feasibility studies or have foreshadowed constructing lithium refineries in Western Australia, including Covalent Lithium (a joint venture of Kidman and SQM), Albemarle, Mineral Resources, Altura, Pilbara and Neometals. Generally, there is no specific classification of processing technologies for spodumene. Some reviews grouped these methods based on the end-product of lithium compounds that form during the digestion, roasting or leaching steps. For example, nomenclature such as sulfation, chlorination, carbonation and fluorination describes methods that produce Li2SO4, LiCl, Li2CO3 and LiF, respectively (Choubey et al., 2016). Other reviews classified the treatment technologies depending on the type of additives such as acid or alkaline processes, referring to treating spodumene with acid and alkaline materials (Meshram et al., 2014). In the current review, we will use the term “digestion” to denote the typical industrial treatment of βspodumene with concentrated H2SO4 at 250 – 300 ºC, and reserve the word “roasting” to represent the heating of α- or β-spodumene with different additives and the term “alkaline leaching” to represent the pressure leaching processes of β-spodumene with solutions of salt of alkaline and alkaline-earth metals at around 200 – 250 ºC. While roasting operations typically proceed at 900 – 1150 ºC, roasting of β-spodumene with mixtures of Na2CO3 with sodium formate or sodium acetate may take place at temperatures as low as 290 – 350 ºC (Peterson, 1960).
2.4.3.3. Lime-gypsum roasting process
The Bureau of Mines in the USA studied the roasting of α-spodumene with gypsum in the presence of lime (Hayes et al., 1950; Sternberg et al., 1946) at a temperature of 1100 – 1150 ºC for 2 – 3 h with a mass ratio of spodumene/limestone/gypsum of 4:4:2, based on the following reaction. 2α-LiAlSi2O6 + 6CaCO3 + 2CaSO4 = Li2SO4 + 4Ca2SiO4 + Al2O3 + 3CO2 (2.3) The authors recommended using only materials that can decompose to produce lime, such as CaCO3 that releases CO2 to produce CaO during heating. Calcium oxide reacts with spodumene, destroying its crystallographic structure. In the experiments, the authors ground the spodumene concentrate with gypsum and lime, then pelletised the ground mixture with a solution of bentonite by tumbling the wet material in a cement mixer. The calcined material required leaching with a solution of CaCl2, to produce high purity LiCl through a series of refinement and purification steps.
The maximum lithium recovery of 90 % necessitated a long heating time of 3 h. Factors such as the long heating time at high temperature, excess additives (i.e., the fraction of α-spodumene in the furnace is only 40 % of the solid mixture) and mechanical pre-treatment make this process economically unfeasible due to lower kiln throughputs and high energy intensity. These are important disadvantages of the lime-gypsum process in comparison to applying Na2SO4 with a small amount of calcium flux in the roasting step, as will be demonstrated in this thesis.
2.4.3.4. Chloride/chlorine roasting processes
The chloride roasting processes rely on the reaction of spodumene with alkali or alkali-earth metal halides such as NaCl, KCl and CaCl2 or with gaseous chlorine gas. Although the reaction is much faster with β-spodumene and requires milder conditions, it can also proceed with αspodumene. It normally requires excess amounts of NaCl, KCl and CaCl2, and temperature in excess of 1000 ºC for α-spodumene, and 800 °C for β-spodumene. Likewise, the reaction with chlorine gas can advance with both α- and β-spodumene, but at significantly slower rates for α-spodumene.
The recovery of lithium from β-spodumene may occur without structural changes and, in this situation, it corresponds to ion exchange. Peterson and Gloss (1959) studied the chlorination of α-spodumene concentrate with potassium chloride at 1000 – 1100 ºC (Peterson and Gloss, 1959). In this study, the produced LiCl sublimed and was collected from the outlet gases. To recover the remaining lithium from the solid residue, it was necessary to treat the residue with a strong acid. In another approach, (Medina and El-Naggar, 1984) deployed a double chloride compound of tachyhydrite mineral (MgCl2-CaCl2 . 12H2O) to produce the water-soluble LiCl during the calcination. To recover 87 % of lithium, the process was operated at 1150 ºC for 2 h and required significant excess of additives (spodumene/additives = 1:8) and large quantities of boiling water (1:50 solid to liquid ratio) over a leaching period of 4 h. Based on XRD measurements, the authors suggested that the melting of CaCl2 induced the high lithium extraction. Figure 2.14 demonstrates that lithium recovery increased from 52 % at 973 K (700 ºC) to 90 % at 1423 K (1150 ºC), but above this temperature, the volatilisation of LiCl resulted in a decreased recovery.
Obtaining high lithium extraction by this process requires high temperature and high amount of additives. The spodumene fraction in the roasting mixture only corresponded to 11 % severely limiting the throughput of Li through the kiln, making the entire process economically infeasible due to its high capex and opex (energy consumption). Furthermore, at high temperature, LiCl sublimes and need to be condensed from exhaust gases as done by Barbosa et al. (2014), who roasted β-spodumene with excess Cl2 gas at 1100 ºC (Barbosa et al., 2014). Collecting the sublimed LiCl, high energy requirements of the thermal treatment, and the requirement for controlled cooling of the flue gases to collect LiCl from the gas phase are factors that make this process impractical on an industrial scale, at present. To resolve the high amount of additives and the loss of lithium, Barbosa et al. (2015) investigated roasting of β-spodumene with CaCl2 in a fixed-bed reactor. Roasting for 2 h at 900 ºC of a solid mixture, characterised by a mole ratio of 2:1 of β-spodumene/CaCl2, resulted
in high lithium extraction of 90 %. Here LiCl was collected as a volatile material from the outlet gases. Although the roasting process operated at a relatively low temperature of 900 ºC, CaCl2, with its melting points of 772 ºC, would challenge the present kill design by possibly sintering the spodumene grains and/or damaging the kiln burner. Together with the energy necessary for the two-stage heating (i.e., calcination of spodumene for phase transformation and roasting of β-spodumene), this technology does not appear to be industrially attractive at present.
Thermodynamics of phase inversion of spodumene: assessment of energy requirements
This study provides a comprehensive calorimetric analysis of the behaviour of spodumene concentrate during its calcination. Spodumene concentrate constitutes the main feedstock for production of lithium chemicals. A high-temperature simultaneous thermal analyser collected the heat-flow data, with subsequent calculations producing the heat capacity and other thermodynamic functions and their dependence on temperature. These measurements also afforded an accurate detection of the phase transition of spodumene concentrate. For the first time, we have analysed in detail the thermal transformation of spodumene concentrate, accurately locating the principal peak of spodumene and an accompanying peak marking the displacive evolution from α-quartz to β-quartz (gangue impurity), with their corresponding characteristic temperatures within a maximum error of ± 3 K. The enthalpy and entropy changes of the transformation of the concentrate amount to 27,400 ± 200 J mol-1 , 21.5 ± 0.4 J mol-1 K -1 , respectively, with the enthalpy of transition of pure α-spodumene to be 28,800 ± 200 J mol-1 . Standard functions of the molar heat capacity of the concentrate, before and after the transition, cover the temperature ranges of 298 – 1209 K and 1394 – 1450 K, respectively. These relationships allowed calculating the variation of the molar enthalpy, entropy and Gibbs energy as functions of temperature. For the temperature segment prior to the phase transition, we observed an excellent agreement between our measurement and the results available in literature. The measurement of heat capacity of the concentrate after the transformation event indicates the formation of a mixture of γ- and β–spodumene. We have determined precisely the minimum energy required for the calcination of spodumene concentrate as 1,530 ± 47 kJ kg-1 and discussed practical implications to industrial processing.
4.2 Introduction
Lithium-containing pegmatites have overtaken brines as feedstock for production of lithium chemicals, especially lithium carbonate and lithium hydroxide; the latter nowadays preferred in manufacturing of lithium ion batteries for electric cars, consumer electronics and energy storage. The state of Western Australia presently supplies more than 50 % of the global demand of lithium from its seven spodumene (LiAlSi2O6) mines, with plans for further expansion. Spodumene ores typically contain 12-30 % of spodumene mineral in association with 22-27 % quartz, 30-50 % feldspar and 3-5 % mica, with a smaller concentration of apatite, tourmaline and beryl, and with the sporadic presence of cassiterite, columbite, monazite, pyrite, pyrrhotite and rutile (Colton, 1957); all abundances are expressed in wt. % in this article. The mineral itself comprises up to 8 % Li2O (Edgar, 1968; Kesler et al., 2012; Vikström et al., 2013). Depending on the deposit and separation techniques, processing of spodumene ores yields either the chemical-grade concentrates with Li2O content of around 6 % or technicalgrade concentrates with abundances of up to 7.6 % Li2O and below 0.2 % Fe2O3, as the one sourced for the present study.
The naturally-occurring spodumene adopts the α-structure (α-LiAlSi2O6), i.e., the stable lowtemperature form (Clarke and Spink, 1969). This phase exhibits a dense and fully ordered monoclinic (C2/c) configuration, resulting in high resistance towards attacks by chemical agents (Rosales et al., 2014). The current modus operandi recovers lithium from spodumene via digestion with concentrated sulfuric acid (Chagnes and Światowska, 2015), which requires initial thermal treatment, i.e., transformation of α-spodumene into more reactive phases that are amenable to acid leaching (Ellestad and Milne, 1950; Tian et al., 2011; White and McVay, 1958). The heat treatment of spodumene concentrate usually occurs in a rotary kiln and results in consumption of large amounts of energy (Garrett, 2004; Metsärinta, 2015; Peltosaari et al., 2016). affecting the economics of lithium refining and its environmental footprint. Spodumene exists in two forms at high temperatures, namely, the β- and the γ-polymorphs.
These polymorphs are less dense than α-spodumene, comprising silicon (Si) and aluminium (Al) atoms randomly distributed in the tetrahedral sites (Li, 1968). However, it is likely that some ordering of Si and Al is present, in line with Löwenstein’s aluminium avoidance principle (Löewenstein, 1954; Moore et al., 2016). The change of the Al3+ coordination from sixfold (αspodumene) to fourfold (both γ- and β-spodumene) modifies the silicate framework of the original pyroxene mineral and allows significant mobility of Li+ ions (Moore et al., 2016; Welsch et al., 2015). The γ-spodumene phase adopts the hexagonal structure of high quartz (P6222), while the β-spodumene exhibits the keatite configuration of P43212 with Al and Si tetrahedrons forming five-fold rings (Li and Peacor, 1968; Moore et al., 2018). These rings facilitate the formation of zeolite-like channels which enable movement of Li+ and contribute to the ion-exchange capability of this phase (Li and Peacor, 1968; Skinner and Evans, 1960; White and McVay, 1958).
Figure 4.1 describes the molecular and crystallographic structural parameters of α-, β-, and γspodumene in which the low temperature (unreactive) spodumene polymorph (α-spodumene) absorbs a particular amount of energy during the heating process to convert to its high temperature (reactive) phases. The aforementioned spodumene polymorphs relate to one another by reconstructive transformations under different conditions (Li, 1968; Sharma and Simons, 1981), characterised by relatively slow kinetics and considerable energy consumption (Putnis, 1992). The thermal mineralogical transformation of α-spodumene occurs above 900 ºC (1173 K) (Deer et al., 1992). Hence, in addition to prior beneficiation to produce the concentrate of α-spodumene, the energy required to achieve the thermal transformation remains a significant cost factor in the lithium extraction from spodumene, in addition to expenses incurred by purchasing the concentrate and the reagents (mainly, concentrated H2SO4, NaOH and Ca(OH)2), as well as disposal of the by-products (mainly, Na2SO4 and aluminosilicates).
Conclusion
This dissertation has described the transition reactions of spodumene, providing new and
effective heating strategies. By measuring the changes in the mineralogy of spodumene
concentrate during the initial fast heating period followed by an extended interval of slow
heating, and by experimenting with finally ground samples, with and without additives, we
determined the reaction pathways and evaluated different calcination and roasting methods for
improved conversion of α-spodumene. Two samples, one relatively crystalline with particles
of d80=120 µm heated slowly (i.e., 10 C min-1
) and the second amorphous sample, finely ground
to d80=20 µm heated at between 30 and 50 ºC min-1
, yielded the same reaction pathway of α→
γ → β-spodumene. The conversion of α-spodumene at 1050 ºC amounted to 30 – 35 % and 75
– 85 % for the 120 and 20 µm particles, respectively. For both samples, γ-spodumene appeared
at an average temperature of 900 ºC. This was the first new crystalline phase identified in the
ex-situ (d80=20 µm) measurements, and its appearance in the spectra from the in-situ
synchrotron XRD (d80=120 µm) measurement was associated with a clear decrease of
amorphous background. This demonstrates that, the formation of γ-spodumene is initiated by
the re-crystallisation of amorphous phase.
Calcination of the 120 µm concentrate at a fast initial heating rate of 100 ºC min-1
resulted in
the formation of a new phase of β-quartz solid solution (β-quartzss) at 875 ºC, while the
appearance of γ-spodumene was delayed to 1025 ºC. Both β-quartzss and γ-spodumene
converted to β-spodumene along two parallel reaction pathways of α → β-quartzss → βspodumene and α→ γ → β-spodumene. Together with a direct conversion of α→ βspodumene, the total conversion of α-spodumene at 1050 ºC corresponded to 60 – 70 %. Thus
the results presented in the thesis described, for the first time, the effect of the amorphicity and
Chapter: 7 Conclusion and Recommendations
216
thermal history of the sample on the transformation reactions, suggesting an effective
conversion strategy for α-spodumene that integrates the mechanical grounding with the thermal
treatment. Building on this newly gained understanding of the conversion reaction and on
measurements of heat capacity, the thesis assesses the energy requirement of practical largescale kilns presently used in lithium refineries.
The thesis also analyses the results from the high-temperature calorimetric measurements of
bulk spodumene concentrate (d80=315 µm) for a temperature range of ~ 25 – 1200 ºC (298 –
1473 K). Two endothermic peaks appeared in the calorimetric traces, one corresponding to the
rapid transformation of quartz at 570 ºC (843 K) within a narrow temperature window of 20
ºC, and the second relating to the slow transformation of α-spodumene at 997 ºC (1270 K) in a
relatively wide temperature range of 180 ºC. Integrating the first peak, and comparing the heat
absorbed for the transformation of quartz with the literature data for this phase change, allowed
us to estimate the concentration of quartz in the spodumene concentrate as around 4 %.
The initial, maximum and final temperatures of the transformation peak of spodumene amount
to 936, 1066 and 1121 ºC (1209, 1339 and 1394 K), respectively. The presence of quartz, of ~
4 wt. % displays negligible effect on the heat required for the phase change of spodumene
concentrate. Prior to the phase change, the calorimetric measurements yielded thermodynamic
functions that agreed well with those available in handbooks (Robie and Hemingway, 1995).
However, our work provided thermodynamic properties of spodumene during and after the
transition, as these data were unavailable in literature. The enthalpy change of the transition
of quartz appears negligible (17.4 J mol-1
), while that of α-spodumene amounted to 27,400 J
mol-1
of concentrate. The thermodynamic properties served to perform an assessment of the
minimum energy requirements for transformation of spodumene, yielding a figure of 1,530 kJ
Chapter: 7 Conclusion and Recommendations
217
kg-1
of concentrate. In comparison to the current industrial energy usage, this implies an excess
of energy in the industrial process of more than 82 %, suggesting possible optimisation of the
operation of industrial kilns. The sluggish transformation of spodumene at high temperatures
observed in calorimetric studies suggested complex reaction kinetics and significant activation
barriers, leading us to undertake a detailed kinetic analysis.
Previous studies adopted approaches that involved the fitting of kinetic models and deriving
simultaneously the values of activation energy (Ea), pre-exponential factor (A) and the kinetic
model itself. Unfortunately, such approaches do not yield unique values of the thermodynamic
triple (i.e., Ea, A and kinetic model). This prompted us to employ the isoconversional analysis
that yields estimates of activation energy as a function of the extent of conversion, with these
estimates being independent of kinetic models. A series of thermo-analytical experiments at
different heating rates (5, 10, 15 and 20 K min-1
) determined the dependency of the activation
energy on the degree of conversion and afforded subsequent estimates of the pre-exponential
factor. The result shows that, both the activation energy and pre-exponential factor vary
strongly with α.
The calorimetric measurements confirm that, the reaction of the transformation of αspodumene does not represent a single step process of the first order, as previously reported.
The isoconversional analysis revealed that, the activation energy decreased monotonically from
892 to 635 kJ mol-1
, with ln(Aα/min) declining from 77 to 55, with the latter trend expected
from the compensation effect. As the variation of the reaction rate with temperature displays
one global maximum without pronounced shoulders, it appears that, consecutive reactions,
rather than parallel reactions, govern the transformation process. The isoconversional model
facilitated prediction of the time required for calcination of spodumene at a constant
Chapter: 7 Conclusion and Recommendations
218
temperature. While, we were unable to gain insights into detailed kinetic mechanisms that
operate in phase transition of α-spodumene, our data demonstrate that, the conversion of αspodumene does not follow a single-step kinetic model of the first order.
Earlier studies demonstrated that, a considerable extraction of lithium from spodumene
concentrate is only possible after heating the concentrate for at least 30 min at 1000 ºC. In
practice, the transformation reaction of spodumene proceeds fast enough only at temperatures
in excess of 1000 ºC. In large-scale industrial processes, the calcination of spodumene
concentrate takes place at temperatures of 1050 – 1150 ºC for up to 2 h. As mentioned above,
this is a highly energy-intensive operation.
Therefore, the second strand of this thesis investigated the recovering of lithium from
spodumene by roasting it at lower temperatures, i.e., in the range of 800 – 900 ºC by mixing
spodumene with CaO and Na2SO4 additives. Roasting of spodumene concentrate with only
Na2SO4 followed by water leaching extracted less than 10 % of the total lithium content. The
residual aluminosilicate adopted the spodumene structure without a significant change.
Likewise, the roasting of spodumene concentrate with CaO yielded the recovery of less than 5
% with water leaching. The structure of the calcine material and residual aluminosilicate
showed the appearance of both γ- and β-spodumene.
Simultaneous application of both CaO and N2SO4 increased the Li recovery to 94 %, compared
to the limited extraction of 90 % without these additives, for the traditional sulfuric acid
digestion process (Francis et al., 2018). The subsequent processing avoided the digestion of
the calcine with sulfuric acid, requiring only the water leach. The process operates optimally
for the following conditions: Na2SO4:CaO:spodumene mass ratio of 0.8:0.01:1.0, roasting
Chapter: 7 Conclusion and Recommendations
219
temperature and time of 882 ºC and 2 h, respectively, solid to liquid ratio of 1:100 (w/v), and
the leaching temperature and time of 50 ºC and 2 h, in that order. The solid to liquid ratio
remains to be optimised. The XRD analyses of the calcine and residue revealed the formation
of the water-soluble salt of LiNaSO4. The roasting step required 18 % more energy per tonne
of processed spodumene. While optimisation of kiln operation remained outside the scope of
our investigation, as such optimisation needs to be performed for the entire refinery, not just
for the kiln in isolation, and include changes to the kiln throughput and residence time of the
feed, as well as removal of the acid-digestion step and adjustments to the purification stage.
With respect to roasting α-spodumene with CaO alone, we obtained energy savings of about
20 % in comparison to traditional calcining of spodumene, as the kiln can function at 800 – 900
ºC rather than at 1100 ºC. This process did not modify the leaching stage with the concentrated
sulfuric acid, and operated at optimum for the ratio of spodumene:CaO of 1:0.1. The extraction
efficiency of lithium amounted to 91 %. We observed no sintering of the reacting mixture; i.e.,
calcine preserved powdery nature of the feed material. Investigation of the fate of calcium and
its possible removal in subsequent purification stage remained outside the scope of our study.
7.2. Recommendations
The scientific studies included in this thesis stimulated a number of important research
questions. The following text discusses the recommendations for future research.
Chapter: 7 Conclusion and Recommendations
220
Combination of mechanical and thermal treatments of spodumene concentrate with particle
sizes of d80=20 μm leads the transition reaction that favours the generation of γ-spodumene at
1050 ºC. This phase has the tetrahedrally-coordinated Al3+ and the less packed structure just
like β-spodumene. Furthermore, it shows high reactivity for lithium extraction in the standard
strong acid treatment (Müller et al., 1988). Developing a process for lithium recovery from γspodumene may result in an important energy saving. Practical energy assessment of the
grinding step should be conducted to compare the energy requirements of this approach with
that involving only the thermal treatment.
The initial fast heating of the crystalline spodumene concentrate (d80=120 µm) showed the
formation of β-spodumene at 925 ºC, reducing the energy of the calcination process, in
comparison to slow heating of the feed. It is therefore recommended to use the same approach
to calcine the fine grain-size sample (d80=20 µm) to investigate its transformation behaviour,
followed by a detailed energy assessment. We also suggest to explore whether flash heating
of ground concentrate offers advantages over kiln heating.
Our calorimetric experiments investigated the thermodynamic and kinetic behaviours of a highgrade spodumene concentrate with particle sizes of d80=315 µm. The measurements enabled
estimation of the energy requirement for the transformation of α-spodumene concentrate as
1,530 kJ kg-1
. Reduction of the particle size (d80=20 µm from d80=120 µm) of the spodumene
concentrate reduced the temperature for the complete conversion to β-spodumene, from around
1200 to 1100 ºC without holding time. While the reduction of the heating temperature
translates into energy savings, the comminution of spodumene concentrate to such small
Chapter: 7 Conclusion and Recommendations
221
particles may offset the energy gain. Therefore, we recommend developing an integrated
energy assessment that includes the combined energy requirement of both heating and grinding.
The fast initial heating of α-spodumene in our in-situ XRD experiments revealed a significant
change in the reaction pathway because of the appearance of the solid solution phase that
displays the β-quartz configuration, which subsequently increased the conversion of αspodumene at lower temperatures. The development of a kinetic model, based on the
isoconversional approach, should yield kinetic parameters of immediate practical importance.
However, detailed understanding of the phase-transition reactions that operate during the
thermal treatment of spodumene, especially the effect of amorphicity, can only come from
detailed microscopic analyses supported by sophisticated quantum chemical modelling, such
as those involving DFT (density function theory) formalism embedded in, say, VASP or DMol3
software packages.
Calcination of spodumene concentrate with CaO induced early transformation of α-spodumene
at 882 ºC. Further work on the thermodynamic assessment (similar to the approach
documented in Chapter 4) should shed more insight into the economic feasibility of this
strategy.
Heating of spodumene concentrate with both CaO and Na2SO4 to improve Li recovery
decreased the fraction of spodumene in the kiln to about 55 %, while the energy requirement
increased only by 18 % per tonne of processed spodumene. As this approach leads to the
removal of the traditional sulfuric acid digestion step, one needs to perform a detailed study on
the overall economics of the entire refinery that takes into consideration the increased cost of
Chapter: 7 Conclusion and Recommendations
222
thermal treatment of the feed (both capex and opex), less expensive downstream recovery of
lithium due to removal of the acid digestion step and possible changes to the purification stage.
Finally, we suggest to investigate a process that includes roasting of α-spodumene with a small
amount of CaO additives followed by pressure leaching with a solution of Na2SO4. Such a
process may offer advantages over that of the present alkaline Na2SO4 pressure leach by
avoiding the addition of CaO to the leaching solution and possible lower operating temperature
and pressure.
7.3. References
Francis, L.-L., Colin, D., Gervaise, S., Nicolas, L., Magnan, J.-F., 2018. Impact of the
impurities on lithium extraction from β-spodumene in the sulfuric acid process. Miner. Eng.,
129, 1-8.
Müller, G., Hoffmann, M., Neeff, R., 1988. Hydrogen substitution in lithium-aluminosilicates.
J. Mater Sci., 23, 1779-1785.
Robie, R.A., Hemingway, B.S., 1995. Thermodynamic Properties of Minerals and Related
Substances at 298.15 K and 1 bar (105 Pascals) Pressure and at Higher Temperatures: U. S.
Geological Survy Bulletin
Thermal Treatment of Spodumene (LiAlSi2O6) for Lithium ExtractionArif A. ABDULLAH, BSc, MSCThis thesis is presented for the degree of Doctor of Philosophy Chemical Engineering
 ODM Daily Inspirational Devotional Messages Bible Verse and Prayers ODM
ODM Daily Inspirational Devotional Messages Bible Verse and Prayers ODM



